Electromagnetic Spectrum
By Ron Hranac
You’ve probably heard the term electromagnetic spectrum before, but have you ever wondered just what it is? I discussed it briefly in my Fall 2020 Broadband Library article “Radio Frequency” (https://broadbandlibrary.com/radio-frequency/), but thought it might be helpful to dig into the topic in a bit more detail.
Let’s start with a definition, this one from NASA: “The electromagnetic (EM) spectrum is the range of all types of EM radiation. Radiation is energy that travels and spreads out as it goes—the visible light that comes from a lamp in your house and the radio waves that come from a radio station are two types of electromagnetic radiation. The other types of EM radiation that make up the electromagnetic spectrum are microwaves, infrared light, ultraviolet light, X-rays and gamma-rays.”
Refer to Figure 1 for a graphical representation of the electromagnetic spectrum. The scale shown in the figure might be a little misleading, but the reality is that the cable industry uses just a tiny portion of the electromagnetic spectrum in its networks. Depending on plant architecture, we use radio frequencies from 5 MHz to around 1 GHz, although some networks operate to as high as 1.2 GHz or 1.8 GHz. In the infrared light part of the electromagnetic spectrum, we use frequencies in our fiber optic cables from about 194 terahertz (THz) to 229 THz, or wavelengths from 1550 nanometers (nm) to 1310 nm. In case you were wondering, a nanometer is a billionth of a meter, or 0.00000003937 inch.
As you look at the graphic in the figure, note that I included frequencies in units of hertz (Hz) as well as wavelengths in units of meters (m). To understand why, it helps to visualize electromagnetic radiation as waves. Electromagnetic waves can be thought of as somewhat analogous to the ripples that occur when you toss a rock into a pond of water. Start by throwing a rock in the water, then count the number of waves per second that pass by a wooden post sticking out of the water. That’s the frequency. One wave passing by the post each second is a frequency of 1 Hz; if a million waves passed by the post each second, that would be a frequency of 1 MHz. Next, measure the distance between adjacent water ripples’ peaks or valleys to determine the wavelength. Of course, electromagnetic waves travel at the speed of light in a vacuum, and somewhat slower in a medium such as coaxial cable, both a whole lot faster than ripples on a pond.
There is a convenient mathematical relationship between frequency and wavelength, the latter represented by the lowercase Greek letter lambda (λ). The following formulas allow easy conversion between frequency in hertz (fHz) and wavelength (in a vacuum) in meters (λm).
fHz = 299,792,458/λm
λm = 299,792,458/fHz
Because of the very high frequencies involved, we almost always describe light in terms of its wavelength rather than its frequency. At even higher frequencies—primarily in the realm of X-rays and gamma rays—physicists prefer to use photon energy in units of electron volt (eV) rather than frequency or wavelength. A dental X-ray, for instance, has a frequency around 3 exahertz (EHz, where 1 EHz is 1,000,000,000,000,000,000 Hz), which corresponds to a wavelength of about 10-10 meters. The photon energy is about 12.4 kilo electron volts (keV).
In the early 20th century wavelength was commonly used instead of frequency to describe radio waves. At some point frequency in cycles per second started to be used, so a frequency that we know today as 1 MHz was 1 megacycle (Mc). Eventually the designation hertz was adopted, in honor of Heinrich Hertz, the physicist who first conclusively proved the existence of electromagnetic waves in the 1880s. Note that when referring to units of frequency, the “h” in “hertz” is lowercase, while the abbreviation is Hz. (Side note: In the accompanying figure I include microwaves with radio waves. Many depictions of the electromagnetic spectrum separate them.)
Amateur radio operators still use wavelength to describe band allocations in the electromagnetic spectrum. For instance, the 80 meter ham band is 3.5 MHz to 4.0 MHz, the 40 meter ham band is 7.0 MHz to 7.3 MHz, the 20 meter ham band is 14.0 MHz to 14.35 MHz, and so on.
We’re accustomed to using frequency instead of wavelength to describe the RF signals in the coaxial cable part of our networks. But what if we used wavelength? A 5 MHz signal’s wavelength would be 59.96 meters, 50 MHz would be about 6 meters, 750 MHz would be 39.97 centimeters (cm), and 1 GHz would be 29.98 cm. Further complicating things: Those are wavelengths in a vacuum, or free-space wavelengths. Wavelength in coaxial cable is different, because velocity of propagation has to be taken into account. For more about that, see Section 20.5 of the operational practice SCTE 270 2021r1, Mathematics of Cable.
When I look at a graph of the electromagnetic spectrum like that shown in the accompanying figure, I sometimes think of it as being similar to the radio dial in a car from the 1950s or ’60s, except covering much more than just the AM broadcast band. Imagine having a radio that could tune from a few tens of hertz to the frequency of light. That still wouldn’t cover the entire electromagnetic spectrum, though, but it would be fun to tune around and see what could be heard.
If you’d like to take a deeper dive into the topic of the electromagnetic spectrum, a good overview can be found at https://en.wikipedia.org/wiki/Electromagnetic_spectrum
The word “radiation” in the NASA definition might have made you think about radioactivity. It’s important to understand the difference between non-ionizing electromagnetic radiation and ionizing electromagnetic radiation. What is ionizing radiation? From Wikipedia: Ionizing radiation, including nuclear radiation, consists of subatomic particles or electromagnetic waves that have sufficient energy to ionize atoms or molecules by detaching electrons from them.
That part of the electromagnetic spectrum including radio waves, microwaves, and extending up to just above visible light is all non-ionizing radiation. The higher photon energy of extreme ultraviolet, X-rays, and gamma rays, however, means those are considered ionizing radiation.
Some of you may have been in the cable industry long enough to remember the concern about calling signal leakage from a cable network “radiation.” Technically it is radiation of radio frequency (RF) signals from a loose connector or similar, but signal leakage applies to RF signals, which are non-ionizing radiation. Still, there was a push in the late 1970s and early ’80s to stop using the term radiation in part because of a possible misunderstanding by the public, and instead call it signal leakage.
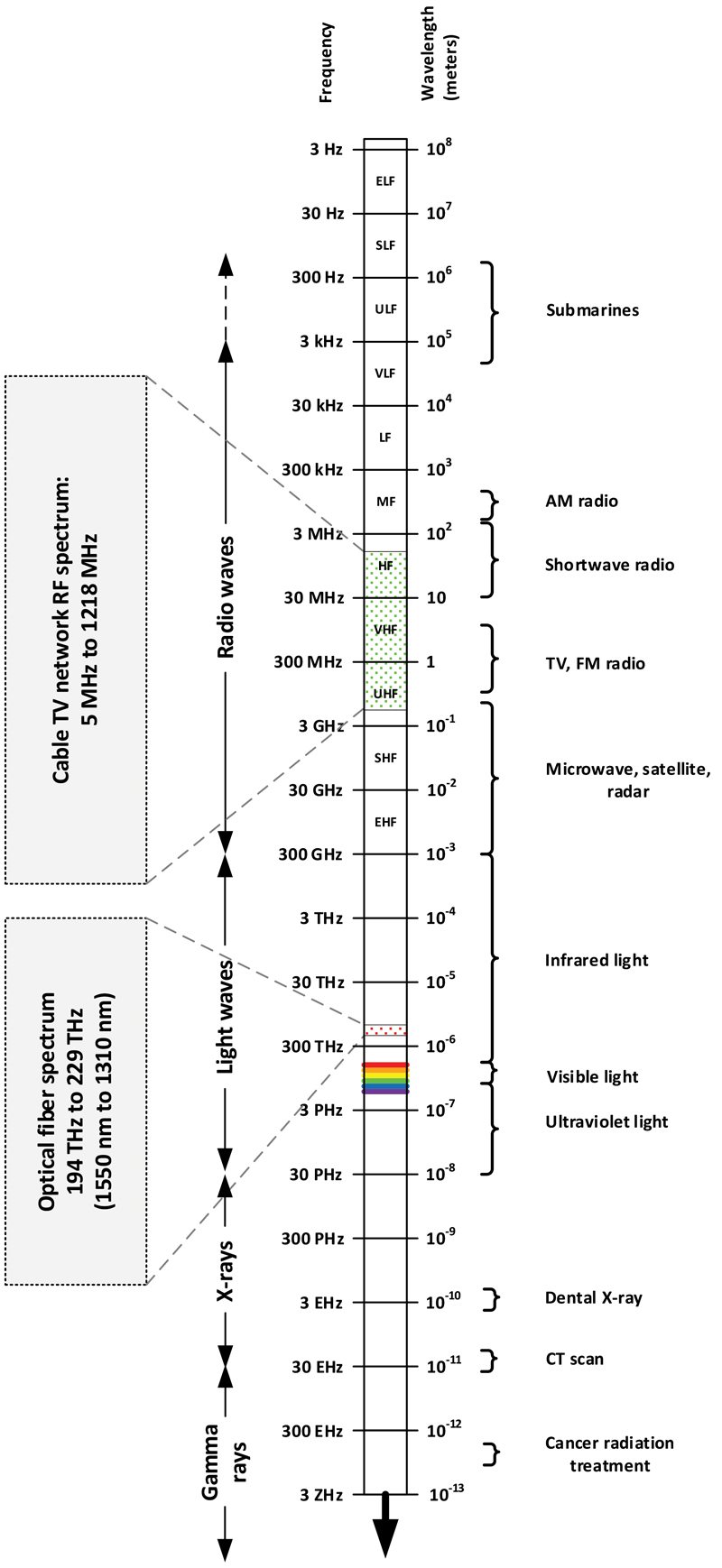
Figure 1. Graphical representation of the electromagnetic spectrum.

Ron Hranac
Technical Editor,
Broadband Library
rhranac@aol.com
Ron Hranac, a 51 year veteran of the cable industry, has worked on the operator and vendor side during his career. A Fellow Member of SCTE and co-founder and Assistant Board Member of the organization’s Rocky Mountain Chapter, Ron was inducted into the Society’s Hall of Fame in 2010, is a co-recipient of the Chairman’s Award, an SCTE Member of the Year, and is a member of the Cable TV Pioneers Class of ’97. He received the Society’s Excellence in Standards award at Cable-Tec Expo 2016. He was recipient of the European Society for Broadband Professionals’ 2016 Tom Hall Award for Outstanding Services to Broadband Engineering, and was named winner of the 2017 David Hall Award for Best Presentation. He has published hundreds of articles and papers, and has been a speaker at numerous international, national, regional, and local conferences and seminars.
Image provided by author.
Shutterstock